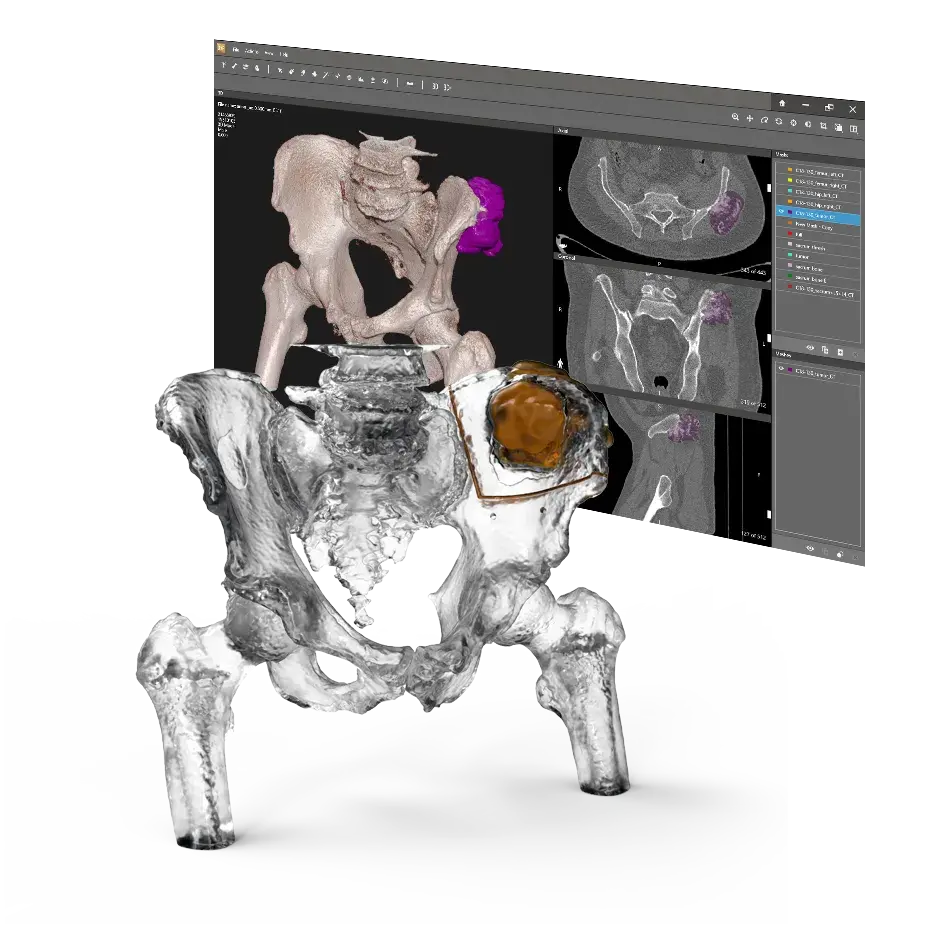
Solutions for Orthopaedics
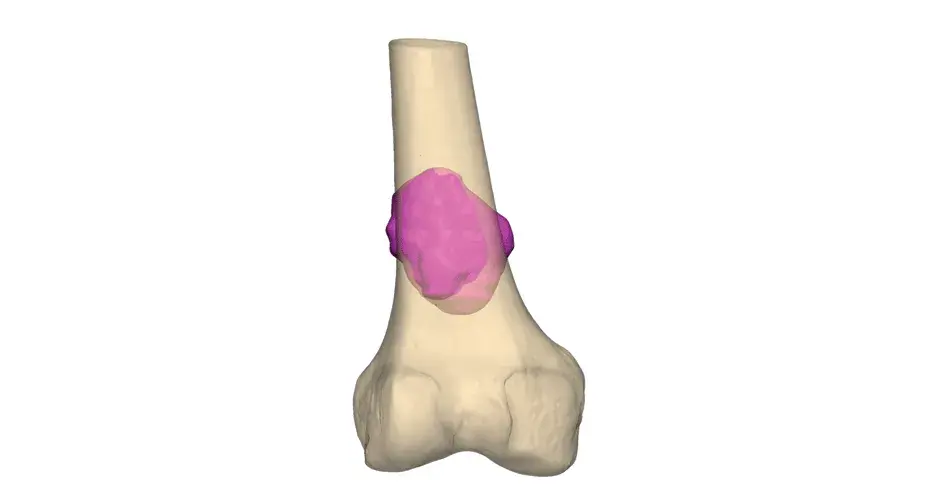
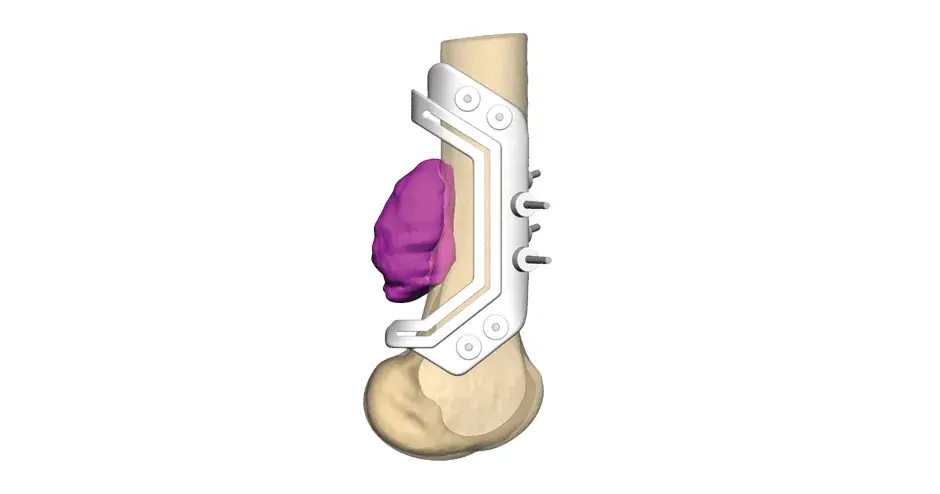
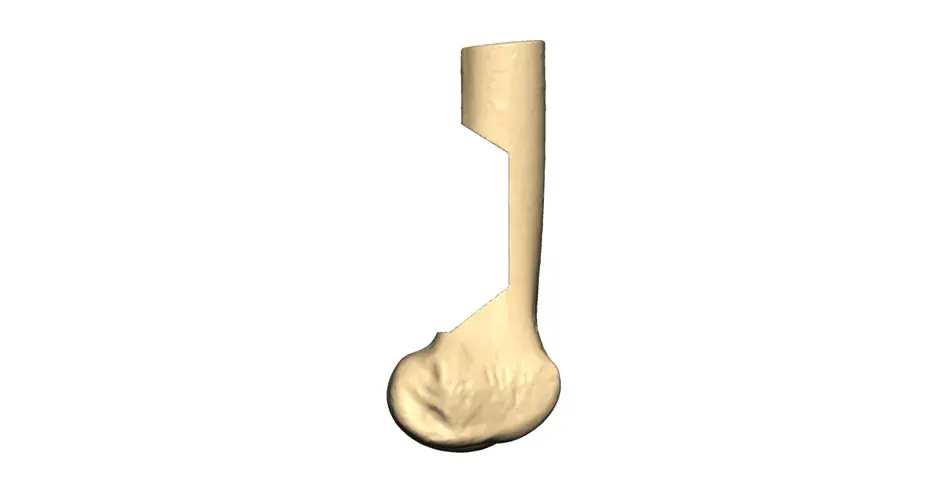
Orthopaedic Oncology Personalized Solutions
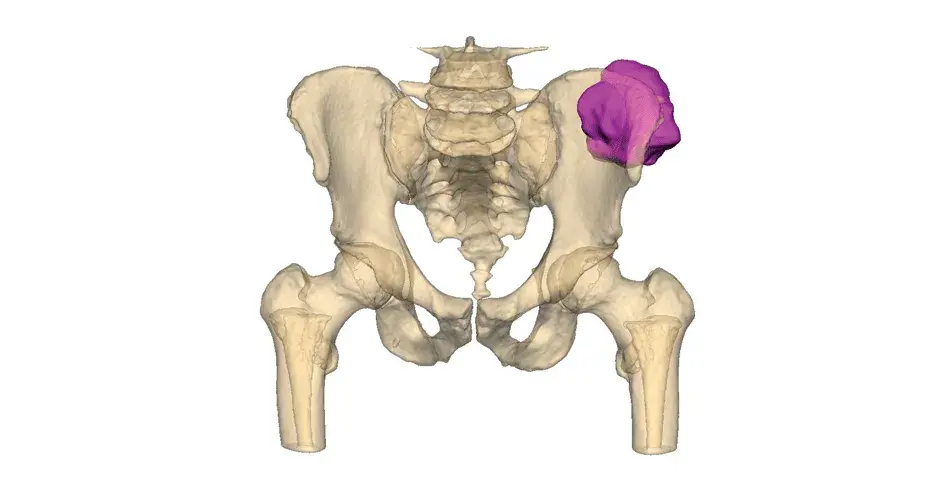
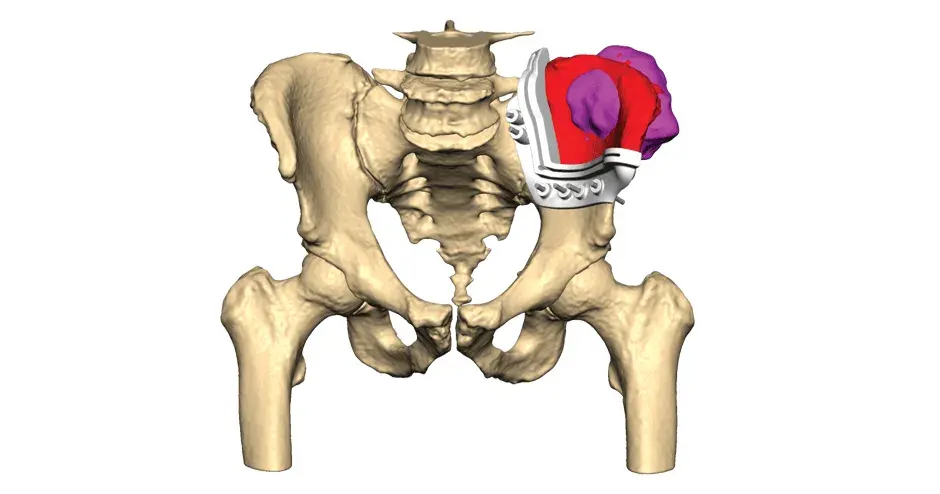
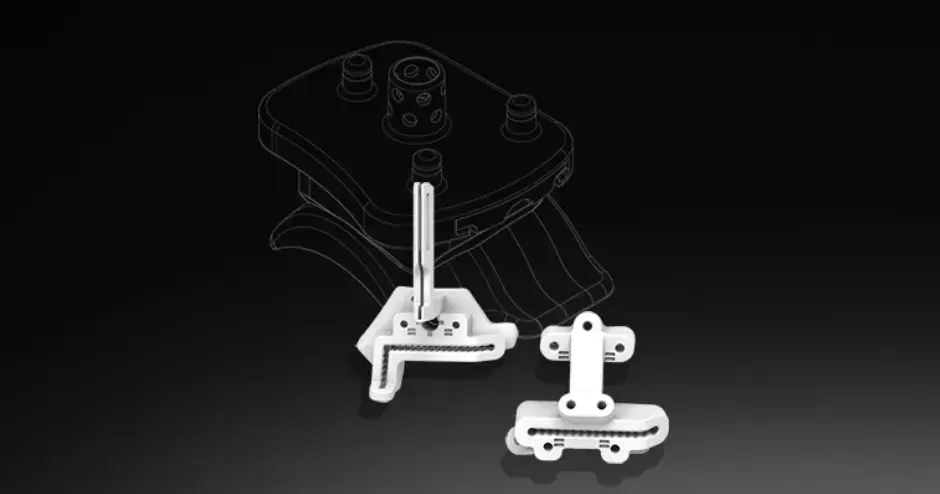
Our VSP Orthopaedics offering is a personalized healthcare solution that was developed specifically for orthopaedic oncology cases. VSP surgical planning solutions provide surgeons with the opportunity to pre-plan surgeries prior to entering the operating room. Based on the surgical plan, patient-matched surgical instruments are designed, and 3D printed for sterilization and use in surgery.
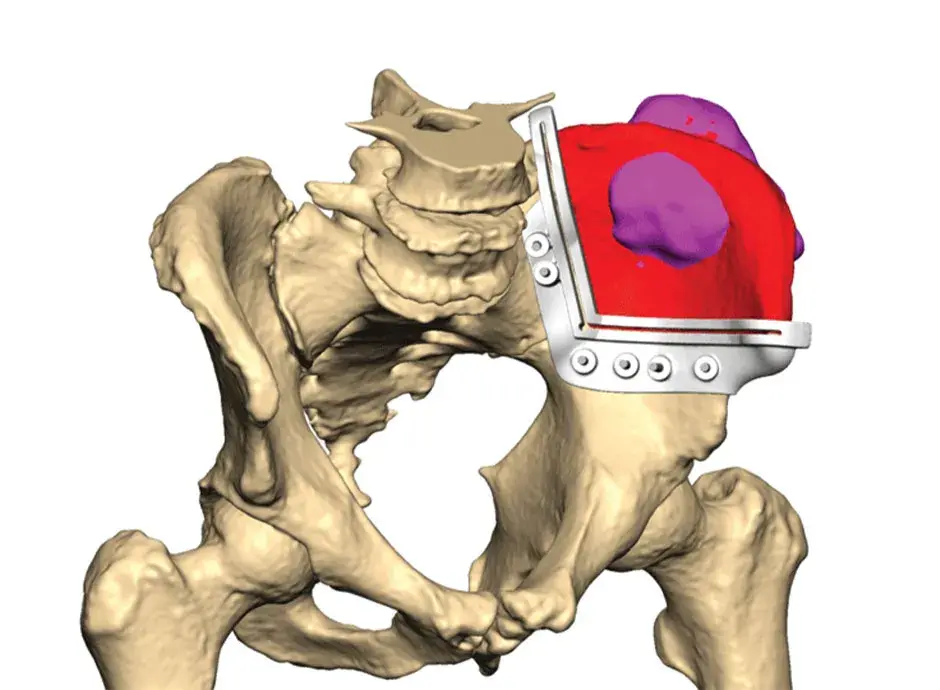
VSP Orthopaedics pelvic tumor resection guide
Extremities
Within the foot and ankle space, 3D Systems has worked with medical device companies to develop VSP surgical planning solutions to compliment off-the-shelf implants. Surgeons are quickley seeing the benefits of a patient-matched instrument that enables precise implant placement. In addition to patient-matched instrumentation, 3D Systems also works with medical device OEM’s to manufacture their implants. We strive to provide an end-to-end solution to improve patient care.
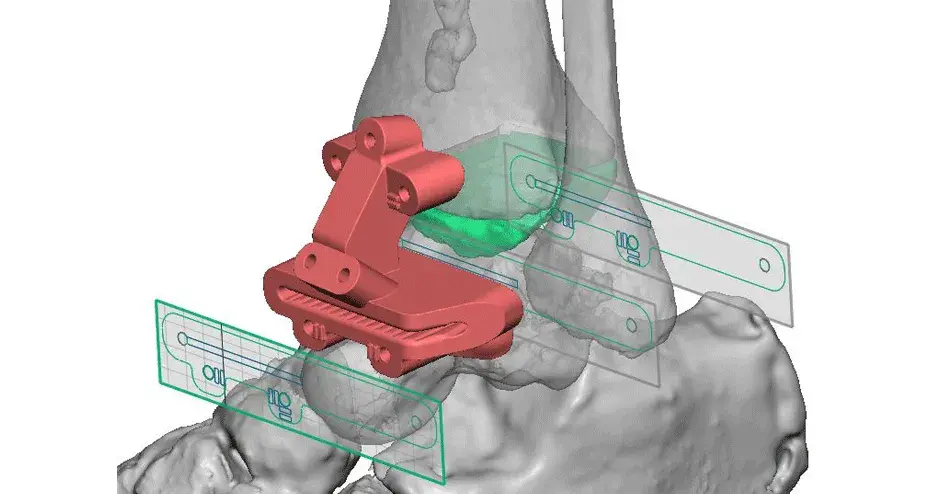
Vantage Ankle PSI
Large Joint
Through our contract manufacturing services, 3D Systems manufactures a range of implants for orthopaedic applications in our ISO-13485 certified facilities. In addition to manufacturing capabilities, experts in the AIG work side-by-side with customers to evaluate their needs and tailor a solution specifically for them.

Additive manufacturing provides accuracy and repeatability for medical device manufacturing
Spine
3D Systems is the trusted manufacturer for the majority of interbody fusion devices on the market today. We work with the leading medical device companies to manufacture their parts and also provide guidance on device design for additive manufacturing and regulatory considerations. Following device clearance, we provide contract manufacturing services at our certified facilities, through our certified partners, and even enable in-house manufacturing.

Interbody fusion devices
Videos
Products for Orthopaedics
DICOM to Print
Advanced Visualization Software
ProJet 7000 HD
Mid-range integrated solution for SLA quality and accuracy
Figure 4 Standalone
Ultra-fast and affordable for same day prototyping and low-volume production
DMP Flex 350
Midsize single laser designed for application development, R&D, and production
DMP Flex 350 Dual
Midsize dual laser designed for application development, R&D, and production
DMP Flex 350 Triple
Midsize 3-Laser workhorse for reliable, repeatable production
DMP Factory 350
Midsize single laser designed for application development, R&D, and production
DMP Factory 350 Dual
Midsize dual laser designed for application development, R&D, and production