With experience manufacturing over two million medical devices, beginning with the very first FDA-cleared 3D printed titanium implant, 3D Systems has world-class, FDA-registered, ISO 13485-certified facilities. These advanced, highly-productive facilities—located in both North America and Europe—have become a preferred choice among medical device manufacturers.
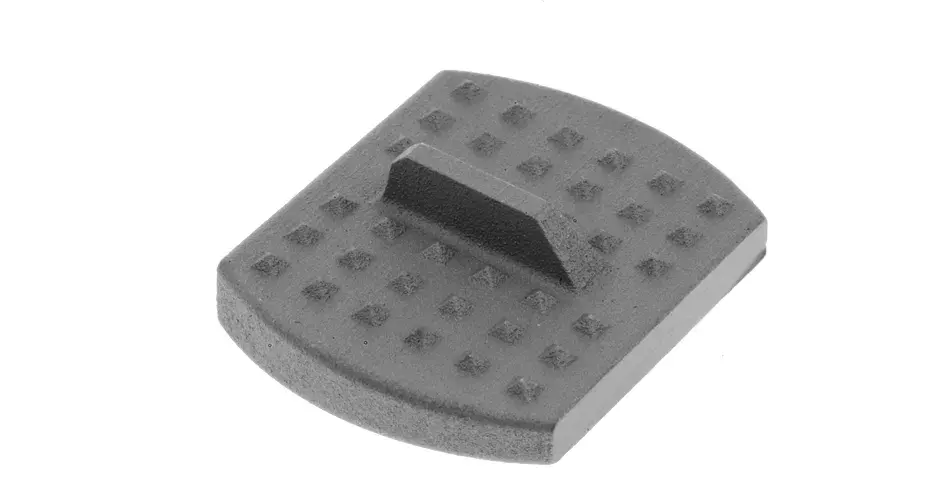
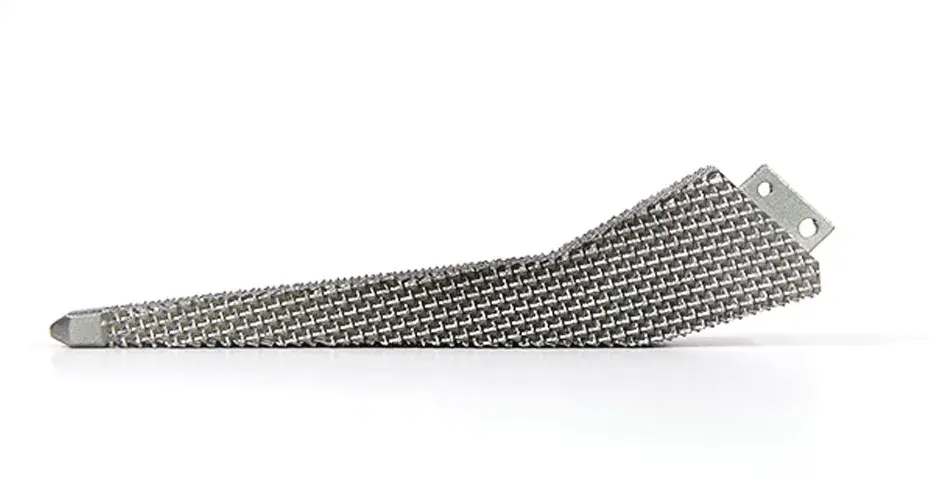
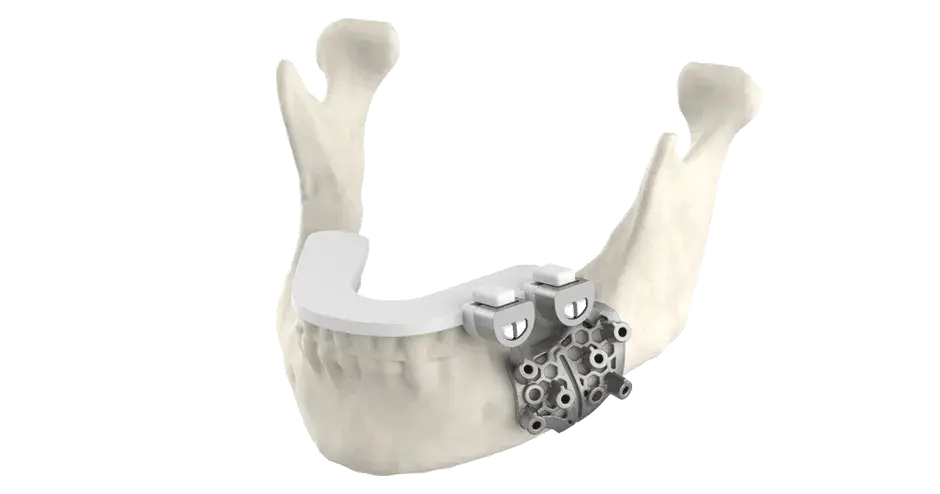
Additive manufactured implants and surgical instruments exhibit excellent mechanical properties enabling accelerated product development and lower inventory costs compared to traditional subtractive manufacturing and its long lead times. Moreover, validated parameter sets enable printing of metallic parts that meet ASTM standards for mechanical properties and chemical composition. The 3D printing process also yields many cost-effective implant types and is fully compatible with traditional (CNC) manufacturing.
3D Systems is the trusted partner of top medical device OEM’s to manufacture their implants and instruments for spine, orthopaedic, extremity, craniomaxiofacial applications and more. Contact us to start your next project.